Diabetes monitoring tech focuses on sharing the care
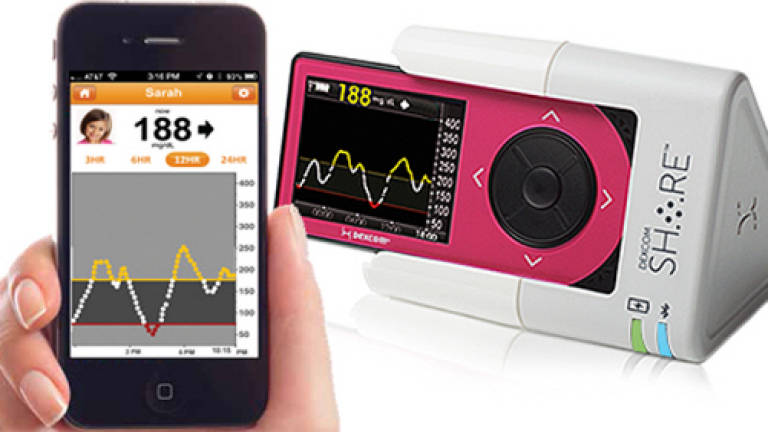
A REMOTE, mobile device that wirelessly transmits patients' continuous glucose monitoring data to up to five caregivers has just been given FDA approval.
Dexcom Share ($299) is not a monitor in itself but a cradle accessory designed to harbour the Dexcom G4 Platinum Continuous Glucose Monitoring System.
Once the Share is attached, those signed up to follow the patient's data can receive alerts on their Apple iPhone or iPod Touch.
Dexcom claims in a press release that its product is the first of its kind and it may well be, although the concept of sharing the care reflects a growing trend in diabetes management.
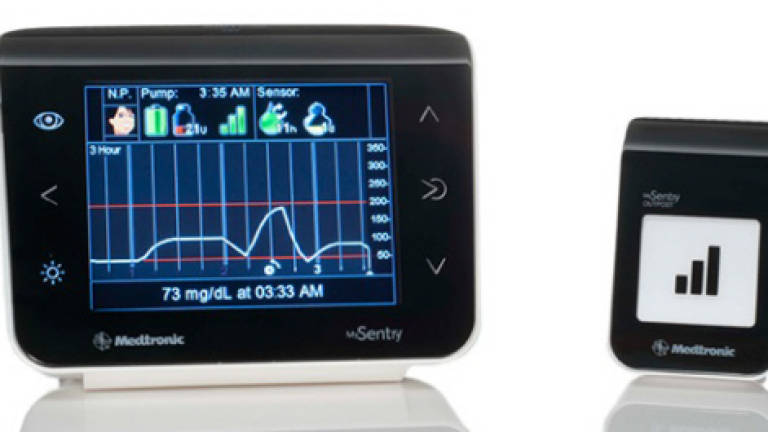
The mySentry continuous glucose monitor is intended for caregivers and parents to watch over loved ones' glucose levels at night, for the bedside Outpost can measure glucose levels from 50 feet away.
Like Dexcom's product, it's a three-party system whose bedside Outpost integrates with the MiniMed Revel System for continuous glucose monitoring (CGM). It received FDA approval in 2012.
Its functioning includes predictive alerts, which can warn up to 30 minutes in advance of abnormal glucose levels.
Gluco-wise is a product in development that will allow for continuous, non-invasive glucose monitoring by sending low-power radio waves through the body, and plans for a data sharing component to ease the worries of parents with diabetic children are detailed on its website.
The company expects to start taking pre-orders in the spring of 2016. – AFP Relaxnews