PETALING JAYA: Mah Sing Group Bhd’s subsidiary, Mah Sing Healthcare Sdn Bhd, has obtained the Certificate of European Union Medical Device Regulation (EU-MDR) for its nitrile examination gloves after meeting the requirements of Regulation (EU) 2017/745.
The group was notified of the certification by its authorised representative, Obelis European Authorized Representative Center, and its product may bear the CE marking and can be marketed in the European Union (EU) and European Economic Area (EEA) territory.
Mah Sing said its product falls under Class I-Devices of the EU-MDR, which requires completion of a technical file and self-declaration of the conformity of the product. The product stipulated in the clearances is nitrile examination powder-free glove (non-sterile).
Mah Sing pointed out that together with the recent issuance of the US Food & Drug Administration 501K and Health Canada Medical Device License, it is ready to accelerate the export of medical-grade gloves to the US, Canada, the EU, and EEA territories.
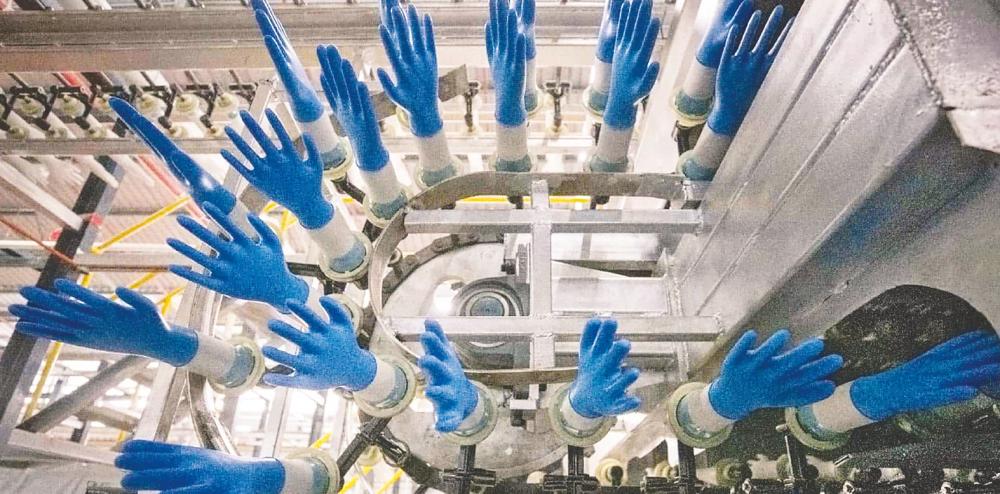
Furthermore, it has completed the commissioning of all 12 production lines and has already received numerous customer sales enquiries. With the added capacity it can produce 38,000 pieces of gloves per production line per hour, translating into a maximum production capacity of up to 3.68 billion pieces of gloves annually.
The group’s factory is a highly automated plant to enhance cost effectiveness and it looks to further improve with auto boxing through a collaboration with packing automation specialists.