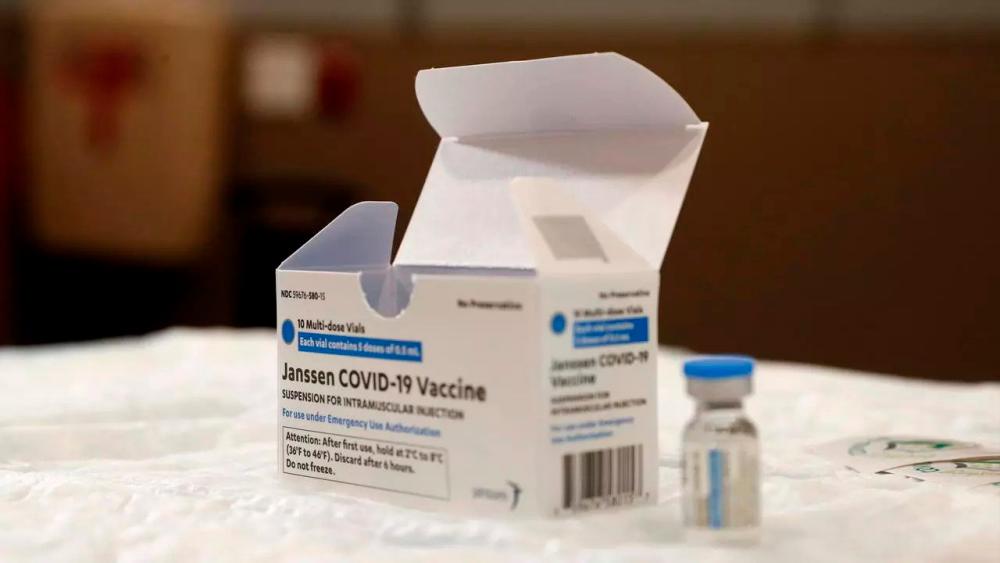
THE HAGUE: The European Union’s medicines regulator approved the single-shot Johnson & Johnson coronavirus vaccine on Thursday, making it the fourth jab to get the green light for the 27-nation bloc.
The decision offers a boost for the EU’s sluggish vaccination programme, even if reports say the first J&J shipments doses may not arrive in European countries until April.
“With this latest positive opinion, authorities across the European Union will have another option to combat the pandemic and protect the lives and health of their citizens,“ European Medicines Agency (EMA) chief Emer Cooke said in a statement.
“This is the first vaccine which can be used as a single dose.”
US pharma giant J&J filed on Feb 16 for approval for the vaccine, developed by its Belgian subsidiary Janssen.
The EU has so far approved three vaccines — Pfizer-BioNTech, Moderna and AstraZeneca-Oxford.
Three other vaccines are under “rolling review” by the Amsterdam-based EMA — Novavax, CureVac and Russia’s Sputnik.
As well as being the first that requires a single injection as opposed to two, the Johnson & Johnson vaccine is easier to store.
The J&J shot however appears less protective than Pfizer and Moderna’s regimes, which both have an efficacy of around 95 percent against all forms of Covid-19 from the classic coronavirus strain.
‘Bumpy’ strategy
The EU has been struggling with a disappointing vaccination rollout that started in January and faltered because of a lack of doses produced by the three suppliers so far.
The head of the EU’s vaccine supply task force, Thierry Breton, said on Tuesday the EU’s “bumpy” vaccine strategy should be augmented by the addition of the Johnson & Johnson jab.
Reported production shortfalls in the United States by Johnson & Johnson would not impact the EU because Brussels had made contingency plans for all vaccines, he said.
“Do not believe that because one company has a problem that overall it will jeopardise the whole programme,“ said Breton, the EU’s industry commissioner.
Europe’s vaccination strategy faced fresh problems on Wednesday as Denmark and Norway said they were temporarily suspending the use of AstraZeneca’s Covid-19 vaccine after some patients developed blood clots.
Norway is not in the EU but is covered by the EMA.
Austria, Estonia, Latvia, Lithuania and Luxembourg have separately suspended the use of a particular batch of AstraZeneca vaccines after a 49-year-old nurse died of “severe blood coagulation problems” in Austria following a jab. — AFP